
Included in the NCCN Guidelines for Prostate Cancer Early Detection.
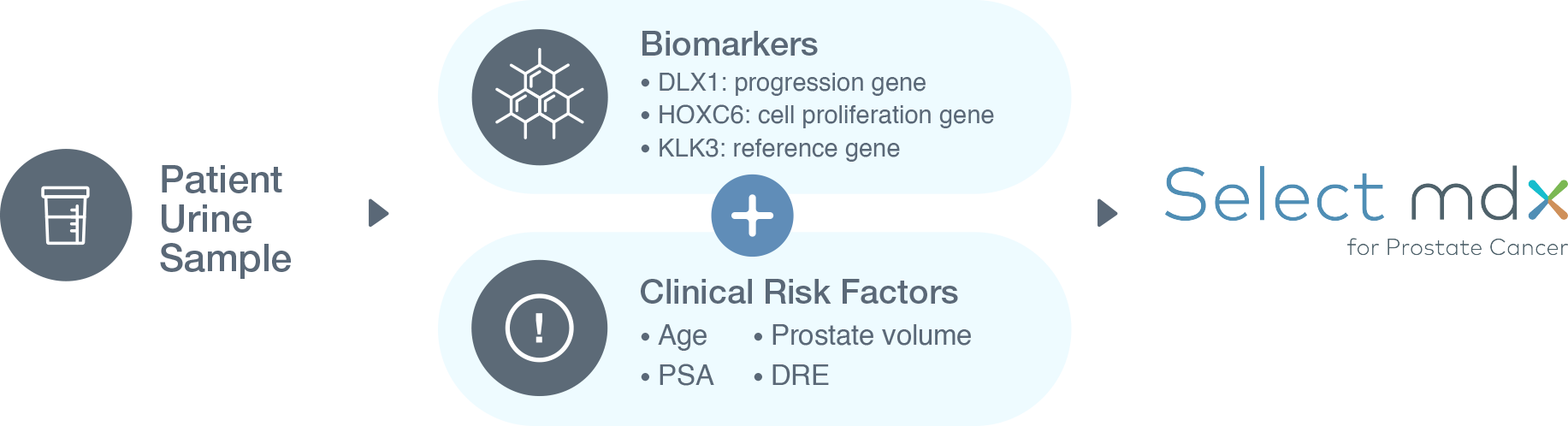
How Select mdx Works
1 in 8 men will be diagnosed with prostate cancer in their lifetime, but not all prostate cancer is aggressive or deadly, and early diagnosis is key. Select mdx fills the gap left by vague and inconsistent screening guidelines, to uncover a patient’s risk for clinically significant prostate cancer. Learn how the Select mdx urine test can reveal your risk.
Select mdx Helps Physicians Determine if a Patient is at Higher or Lower Risk for Prostate Cancer and Which Men Can Safely Avoid Biopsy

A non-invasive urine test (“liquid biopsy”), Select mdx measures the expression of two mRNA cancer-related biomarkers (HOXC6 and DLX1). 1 The test provides binary results that, when combined with the patient’s clinical risk factors, help the physician determine whether:
- The patient may benefit from a biopsy and early prostate detection, or
- The patient can avoid a biopsy and return to routine screening
Overcomes Historical Prostate Cancer Screening Challenges for More Effective and Efficient Diagnosis
Concerns about identifying patients with indolent prostate cancer and subsequent overtreatment have led to recommendations for eliminating the PSA test. An elevated PSA result could be caused by factors other than cancer, including infection, inflammation or benign prostatic hyperplasia.
An elevated PSA is considered the best risk stratifier for the early detection of prostate cancer, resulting in an increased likelihood for curative treatment. In contrast, delayed diagnosis can lead to poorer outcomes, lower quality of life, and higher healthcare costs. 2
Select mdx Increases Physician Confidence in Biopsy Decisions
Included in the National Comprehensive Cancer Network (NCCN) Guidelines
• Biomarkers can improve the specificity of screening methods for clinically significant cancer.
• Included in the 2020 NCCN Guidelines for Prostate Cancer Early Detection.
The clinical utility of Select mdx for Prostate Cancer is well-established:
- Men identified by the test as having a high likelihood of clinically significant cancer can, upon biopsy, be diagnosed and treated sooner, while men identified at very low risk may avoid biopsy
- The test’s negative predictive value (NPV) is 95%, meaning if the test identifies a very low risk, the physician and patient can be 95% sure the patient does not have Gleason score ≥7 (GS≥7) prostate cancer and avoid a biopsy.1
- The test has a very high predictive accuracy (AUC 0.85) for high-grade prostate cancer, which is significantly better than the Prostate Cancer Prevention Trial (PCPT) risk calculator version 2.1
Mdxhealth is regulated under the Clinical Laboratory Improvement Amendments (CLIA) and College of American Pathologists (CAP) as an accredited laboratory to perform high complexity clinical testing. The Select mdx for Prostate Cancer test was developed, and its performance characteristics determined by mdxhealth. It has not been reviewed by the U.S. Food and Drug Administration. The FDA has determined such clearance or approval is not necessary. The test is intended for use as an aid to clinicians for patient management decisions about the need for a prostate biopsy in men with clinical risk factors suggesting an increased risk for prostate cancer. Use outside of this indication has not been validated by mdxhealth. Test results should be interpreted in conjunction with other laboratory and clinical data available to the clinician and relevant guidelines on the decision for biopsy. CLIA# 05D2033858; CAP# 8015399
Contact a Local Representative
Questions about our tests or ready to set up a new account?
How to Order Select mdx
Ordering Select mdx for Prostate Cancer is simple and easy.
Physician Portal
Secure mdx is a safe and secure HIPAA compliant tool that gives you access to patient reports online.
Numerous Studies Verify the Test’s Validation, Clinical Utility and Cost Savings
Multicenter Study of 1,955 Men
Multicenter Optimization and Validation of a 2-Gene mRNA Urine Test for Detection of Clinically Significant Prostate Cancer Prior to Initial Prostate Biopsy.
Study of 144 Men Who Might Benefit from mpMRI
Assessment of an Established TRUS and a Urinary Biomarker-Based Risk Score as an Inclusion Criteria for Multiparametric MRI to Detect Clinically Significant Prostate Cancer
Cost-Effectiveness of Urinary Biomarker Panel in Prostate Cancer Risk Assessment
Select mdx Increases QALY and Cost Savings
Study Involving 418 Men Undergoing Evaluation for Initial Biopsy
Select mdx Impacts Prostate Biopsy Decision Making in Routine Clinical Practice
Clinical, Analytical, Cost Effectiveness Studies
1 Haese, A, et al. (2019) Multicenter Optimization and Validation of a 2-Gene mRNA Urine Test for Detection of Clinically Significant Prostate Cancer Prior to Initial Prostate Biopsy. J Uro. doi: 10.1097/JU.0000000000000293
2 Govers TM, et al. (2018) Cost-Effectiveness of Urinary Biomarker Panel in Prostate Cancer Risk Assessment. J Urol. doi: 10.1016/j.juro.2018.07.034
3 Govers TM, et al. Cost-effectiveness of SelectMDx for prostate cancer in four European countries: a comparative modeling study. Prostate Cancer and Prostatic Diseases. doi: 10.1038/s41391-018-0076-3
4 Trooskens G, et al. (2018) Robust performance of a Urinary Molecular Biomarker–Based Risk Score to detect High-grade Prostate Cancer using optimized cascading models. In: Global Congress on Prostate Cancer; 2018 Jun 28-30; Frankfurt, Germany.
5 Shore N, et al. (2018) SelectMDx Impacts Prostate Biopsy Decision-making in Routine Clinical Practice. Urology Practice. doi: 10.1016/j.urpr.2018.09.002.
6 Trooskens G, et al. (2018) Assessment of an established TRUS and a urinary biomarker-based risk score as an inclusion criteria for multiparametric MRI to detect clinically significant prostate cancer. In: Global Congress on Prostate Cancer; 2018 Jun 28-30; Frankfurt, Germany.
7 Van Neste L, et al. (2016) Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score. Eur Urol, Nov; 70(5): 7 40-7 48.
8 Hendriks RJ, et al. (2017) A urinary biomarker-based risk score correlates with multiparametric MRI for prostate cancer detection. The Prostate, 77(14):1401-1407.
9 Hessels D, et al. (2017) Analytical validation of an mRNA-based urine test to predict the presence of high-grade prostate cancer. Translational Medicine Communications, 2:5. doi: 10.1186/ s41231-017-0014-8.
10 Dijkstra S, et al. (2017) Cost-effectiveness of a new urinary biomarker-based risk score compared to standard of care in prostate cancer diagnostics – a decision analytical model. BJU Int, 120(5):659-665. doi: 10.1111 /bju.13861.
11 Alinezhad S, et al. (2016) Validation of Novel Biomarkers for Prostate Cancer Progression by the Combination of Bioinformatics, Clinical and Functional Studies. PLoS ONE, 11 (5): e0155go1. doi: 10.1371/journal.pone.0155901.
12 Leyten GH, et al. (2015) Identification of a Candidate Gene Panel for the Early Diagnosis of Prostate Cancer. Clin Cancer Res, 21 (13):3061-70.
13 Vinarskaja A, et al. (2011) DNA Methylation and the HOXC6 Paradox in Prostate Cancer. Cancers, 3:3714-3725. doi: 10.3390/cancers3043714.
Posters & Abstracts
- Validation of a two-gene mRNA urine test for detection of high-grade prostate cancer in German men.
- SelectMDx versus Prostate Health Index in the identification of high-grade prostate
- Cost-effectiveness of a two-gene urine biomarker assay in MRI strategies for the initial detection of prostate cancer.
- AUA 2022 Best Posters: Select mdx and Multiparametric Magnetic Resonance Imaging Improve the Diagnostic Workup for the Detection of Prostate Cancer in Biopsy-naive Men
References
1 Haese A, et al. (2019) Multicenter Optimization and Validation of a 2-Gene mRNA Urine Test for Detection of Clinically Significant Prostate Cancer before Initial Prostate Biopsy. J Urol. 2019 Aug;202(2):256-263
2 Govers TM, et al. (2018) Cost-Effectiveness of Urinary Biomarker Panel in Prostate Cancer Risk Assessment. J Urol. doi: 10.1016/j.juro.2018.07.034

















